2025届湖北省腾云联盟高三12月联考物理试题(PDF版,含答案)
文档属性
| 名称 | 2025届湖北省腾云联盟高三12月联考物理试题(PDF版,含答案) |  | |
| 格式 | |||
| 文件大小 | 3.4MB | ||
| 资源类型 | 教案 | ||
| 版本资源 | 通用版 | ||
| 科目 | 物理 | ||
| 更新时间 | 2024-12-12 13:18:07 | ||
图片预览

文档简介
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
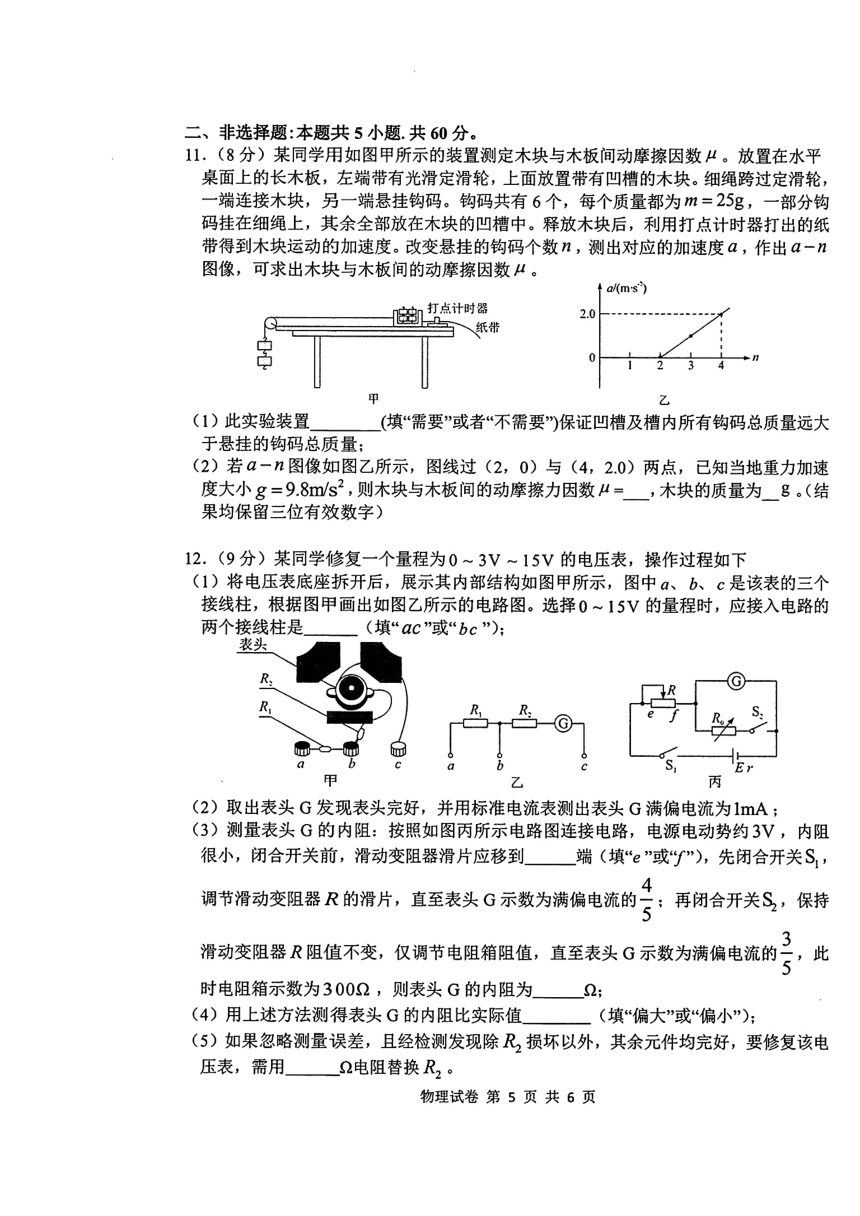
11.(8分 ) 不需要 (2分 ) 0.204 (3分) 145 (3分 )
12. (9分 ) (1) ac (2分 )
100 (2分 )
(4) 偏小 (2分 )
(5) 2900 (2分 )
13.(10分解:(1)气) 体作等容变化,开始时的压强p=1.2p , 温度T=360K,
降温后的压强P =P 温度T =
由查理定律得 2分
T =300K 1分
气体温度降低△ l=T -T =60K =60°C 2分
(2) 设放出的气体先收集起来,并保持压强与保持罐内相同,以全部气体为研究对象,
放气过程:为等p温Y 膨p胀过Y 程,设膨胀后气体的总体积为V。 由玻意耳定律得 = 解得 V=1.2 V
则剩余气体的质量与原 来总质量的比值为:
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
11.(8分 ) 不需要 (2分 ) 0.204 (3分) 145 (3分 )
12. (9分 ) (1) ac (2分 )
100 (2分 )
(4) 偏小 (2分 )
(5) 2900 (2分 )
13.(10分解:(1)气) 体作等容变化,开始时的压强p=1.2p , 温度T=360K,
降温后的压强P =P 温度T =
由查理定律得 2分
T =300K 1分
气体温度降低△ l=T -T =60K =60°C 2分
(2) 设放出的气体先收集起来,并保持压强与保持罐内相同,以全部气体为研究对象,
放气过程:为等p温Y 膨p胀过Y 程,设膨胀后气体的总体积为V。 由玻意耳定律得 = 解得 V=1.2 V
则剩余气体的质量与原 来总质量的比值为:
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
{#{QQABIQgUoggAAhAAARhCEwHCCgKQkhEACQgOwFAEsAAAyAFABAA=}#}
同课章节目录